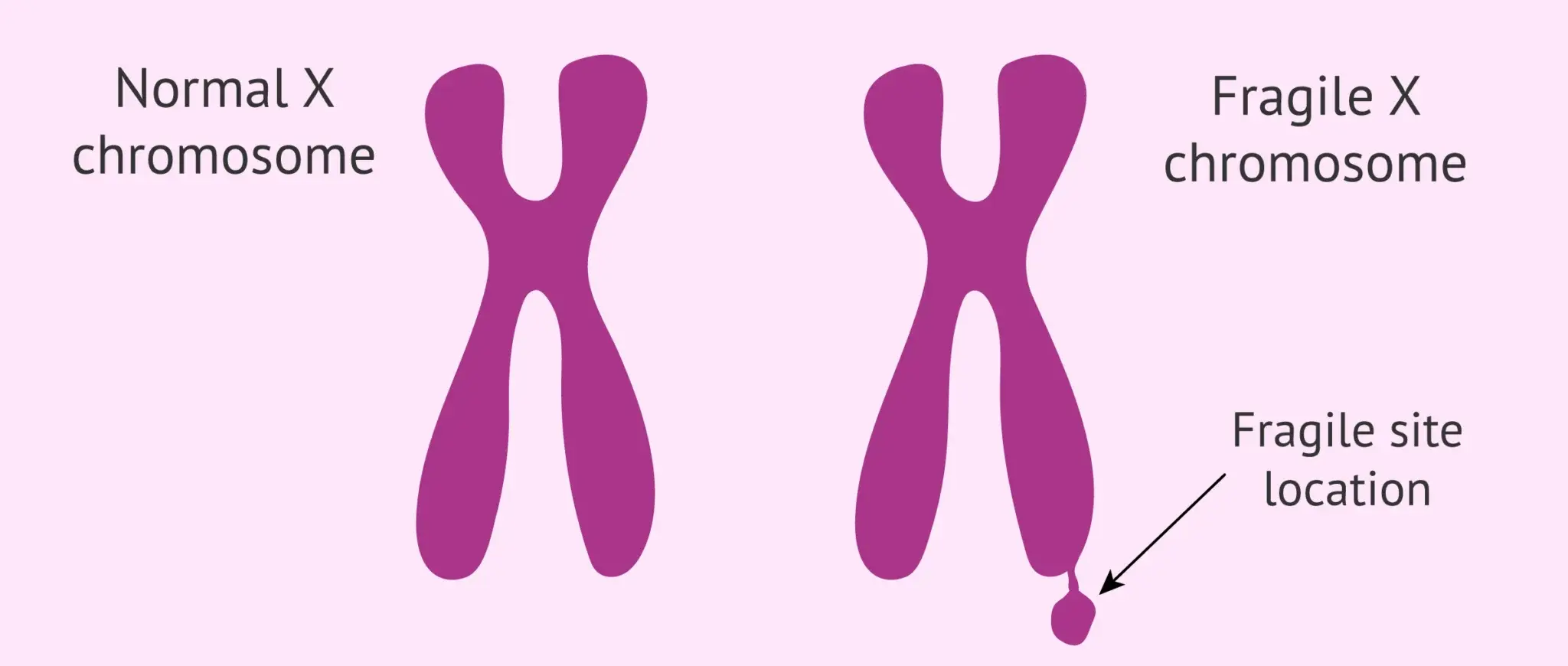
Lenire Biosciences
Lenire Biosciences is a privately held biopharmaceutical company focused on developing novel treatments for Fragile X Syndrome (FXS) and related disorders. FXS is a genetic condition that causes developmental delays, learning and behavioral difficulties, physical abnormalities, anxiety, and symptoms associated with autism spectrum disorder. Lenire's primary therapeutic candidate is VSN16R, an orally bioavailable compound that modulates large-conductance, calcium-activated potassium (BK) channels.
VSN16R was developed in the laboratory of Professor David Selwood at University College London (UCL). It has completed phase II human trials for treating muscle stiffness and involuntary muscle spasms in multiple sclerosis. Lenire Biosciences licensed VSN16R from UCL to develop it as a treatment for FXS, with preclinical studies showing promising results in rescuing FXS-related phenotypes in a mouse model.
The company's approach is based on extensive research into the role of BK channel activity in FXS. The goal is to develop a disease-modifying treatment that can improve many of the core symptoms of FXS, such as anxiety and hyperactivity, which are often resistant to traditional medications.
Lenire Biosciences Completes License Agreement with University College London
Lenire Biosciences Completes License Agreement with University College London for VSN16R, A Novel Clinical Candidate Treatment for Fragile X Syndrome.
(SAN DIEGO, CA, June 16, 2022)
Lenire Biosciences Inc., a biopharmaceutical company developing novel treatments for Fragile X Syndrome (FXS) and related disorders, today announced that it has agreed to a license from UCL (University College London) for VSN16R, a small molecule positive modulator of large-conductance, calcium-activated potassium (“BK”) channels, as a novel therapy for FXS. The development of this technology was supported by UCL Business (UCLB), the commercialization company of UCL.
FXS is a rare, inherited condition that can cause issues including developmental delays, learning and behavioural difficulties, physical abnormalities, anxiety, attention or hyperactivity disorders and autism spectrum disorder. FXS has been detected in all populations and ethnic groups and recent estimates suggest a prevalence of approximately 1 in 4,000 males and 1 in 8,000 females have FXS.
VSN16R is an orally bioavailable compound based on anandamide, a natural signalling molecule in the endocannabinoid system in humans. It was developed in the laboratory of Professor David Selwood (Wolfson Institute for Biomedical Research, UCL), as part of a study that has completed phase II human trials for muscle stiffness and involuntary muscle spasms in multiple sclerosis, a common symptom known as spasticity. A large and diverse set of preclinical studies have implicated deficient BK channel activity in the etiology of FXS, and recent studies in a mouse model of the syndrome demonstrated efficacy of VSN16R in rescuing FXS-related phenotypes. Lenire plans to develop VSN16R to treat patients with FXS.
FXS is the most prevalent inherited form of intellectual disability and a leading cause of autism spectrum disorder. There are currently no disease-modifying therapies for FXS, and some of the most problematic symptoms, such as anxiety, are largely refractory to medications that would be used to treat them in the neurotypical population.
Dr. Craig Erickson, Professor of Psychiatry at Cincinnati Children’s Hospital Medical Center and Director of the Cincinnati Fragile X Research and Treatment Center commented, “We are excited to collaborate with Lenire to test VSN16R in patients with FXS.”
Professor David Selwood commented "The effect of our drug in the disease models is transformative; we are very excited to see this drug go forward to the clinic in people with Fragile X."
About Lenire Biosciences
Lenire Biosciences is privately held, clinical stage biopharmaceutical company that is developing novel, patent protected therapeutics for the treatment of Fragile X Syndrome (FXS), a leading cause of intellectual disability world-wide that also presents with anxiety, attention deficit, hyperactivity, autism spectrum behaviors and other disabling symptoms.

Our mission is to improve patients’ lives with novel pharmacological approaches based on our unique perspective on the core molecular and neurophysiological dysfunctions of FXS and the etiology of its individual symptoms.

Fragile X syndrome, an “orphan disease” affecting approximately 1 in 4-5000 males and 1 in 6-8000 females, is the leading inherited cause of intellectual disability.
Affected individuals also present with severe anxiety, attention deficit, hyperactivity, and other disabling symptoms. In fact, FXS is a leading known cause of autism and it is widely held that treatments that show efficacy in FXS will also have benefits in other conditions on the autism spectrum.