Chromogenic Assay
Chromogenic assays result in a colored reaction product that absorbs light in the visible range. The antigen-antibody complex formed on the solid carrier is separated from other substances by washing. The antibody was labeled by enzyme. After the substrate of the enzyme was added, the substrate becomes a colored product under catalysis of the enzyme-catalyzed, which is directly related to the amount of test substance. The optical density of the reaction product is typically proportional to the amount of analyte being measured. Due to the very high efficiency of the enzyme, the reaction can be greatly enlarged with a high sensitivity.
Principle of optical absorption
Lambert-Beer Law: logI0 / I = ε·C ·b. Absorbance also called optical density values (OD) is proportional to the concentration.
The two most popular enzymes labeling the antibodies are alkaline phosphatase (AP) and horseradish peroxidase (HRP)
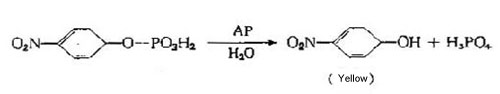
1 AP
Alkaline phosphatase extracted from Escherichia coli is approximately double the size of peroxidase (MW ~80kD) with optimum pH of 8.0. The MW of calf intestinal extracted AP is 100kD and the optimal pH is 9.6.This means that one will typically see a lower enzyme to antibody conjugation ratio. It also means that the larger molecular size of alkaline phosphatase can cause steric hindrance issues due to close packed antigen-antibody complexes. This can result in lower activity than expected for the estimated number of bound enzyme molecules (which is sometimes considered responsible for the "high dose hook" phenomenon).
Alkaline phosphatase is slightly more expensive than peroxidase, but is considered to be more stable. Substrates for alkaline phosphatase range from soluble to insoluble; many can be signal enhanced to increase sensitivity. The most common substrate that produces an insoluble reaction product is BCIP/NBT (5-bromo- 4-chloro-3-indolyl-phosphate/nitroblue tetrazolium). It is recognized as the most effective substrate for immunoblots due to its stability and resistance to fading when exposed to light. The most widely used substrate that produces a soluble reaction product is p-NPP (p-nitrophenylphosphate).
It produces an intense yellow color measurable at 405 to 410 nm. An advantage of this substrate is that it can be allowed to develop for extended periods to obtain a corresponding increase in sensitivity. Normally p-NPP has a slow reaction rate which should be allowed 30 to 60 minutes to reach optimal color development before being stopped with 1N NaOH. It is not recommended for kinetic analysis.
The major disadvantage associated with using alkaline phosphatase is that it is inactivated by chelating agents, acidic pH (< 4.5), or inorganic phosphates. This means that buffers must be specific for alkaline phosphatase, and one cannot use standard assay phosphate buffered saline 2 solutions as diluents or wash solutions that come in contact with the enzyme during an assay. However, chelators (EDTA) and acidic pH are typically used as convenient and inexpensive stopping reagents for alkaline phosphatase reactions
Properties: low background; high sensitivity; but more expensive
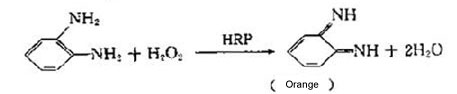
2 HRP
Peroxidase is a glycoprotein with sugar about 18% (MW~44kD) that can usually be conjugated o an antibody in a 4:1 ratio. It is a combination of a porphyrin protein composed of a apoenzyme (enzyme) and a prosthetic group (heme).The apoenzyme is a colorless glycoprotein with a maximum absorption peak at 275 nm. The prosthetic group is a dark brown iron porphyrin ring and has a maximum absorption peak at 403 nm.
Due to its small size, it rarely causes steric hindrance problems with antibody/antigen complexes bound on a surface. Peroxidase is very inexpensive compared to alkaline phosphatase. Several substrates, yielding either soluble or insoluble reaction products, are commercially available for peroxidase. Since all peroxidase reactions require hydrogen peroxide, purchasing commercially available substrates is recommended because these preparations contain stabilized hydrogen peroxide which adds to their value and usefulness.
The major disadvantage associated with peroxidase is that it is incompatible with many preservatives, such as sodium azide, that are used to reduce microbial contamination in many biological buffer solutions. Sodium azide, even in low concentrations, inactivates peroxidase activity. Other compounds or elements that interfere with peroxidase activity are metals found in water and endogenous peroxidases found in biological specimens. These disadvantages can be overcome by using sterile buffers without preservatives, using reagent grade type II water, and pretreating specimens suspected of having high peroxidase levels with hydrogen peroxide prior to use in an assay. Typically, nonbound biological components are washed away prior to the addition of the enzyme, so endogenous peroxidase activity is usually not an issue.
The three most common substrates that produce an insoluble product are TMB (3,3',5,5' tetramethylbenzidine),DAB (3,3',4,4' diaminobenzidine), and 4CN (4-chloro-1-naphthol). The most common substrates that produce soluble reaction products are TMB (dual function substrate), ABTS (2,2'-azino-di [3-ethylbenzthiazoline] sulfonate), and OPD (o-phenylenediamine). TMB is a highly sensitive substrate. Due to its rapid reaction rate, it is ideally suited for on-line kinetic analysis. It produces a blue color measurable at a wavelength of 650 nm. TMB can also be used in endpoint assays by stopping the reaction with 1M phosphoric acid.
A yellow reaction product is formed upon acidification that is measurable at 450 nm. ABTS is considered an all-purpose substrate. Although it is less sensitive than either TMB or OPD, it has the widest working range of any substrate currently available for peroxidase or alkaline phosphatase. The reaction product for ABTS is a blue-green compound measurable at 405 to 410 nm.
Its reaction rate is suitable for endpoint assays and is easily stopped with 1% sodium dodecyl sulfate (SDS), which does not change the color or the absorbance of the reaction product. OPD was once the most popular substrate for peroxidase. It is slightly less sensitive than TMB. Its reaction product is yellow and can be read at 490 nm.